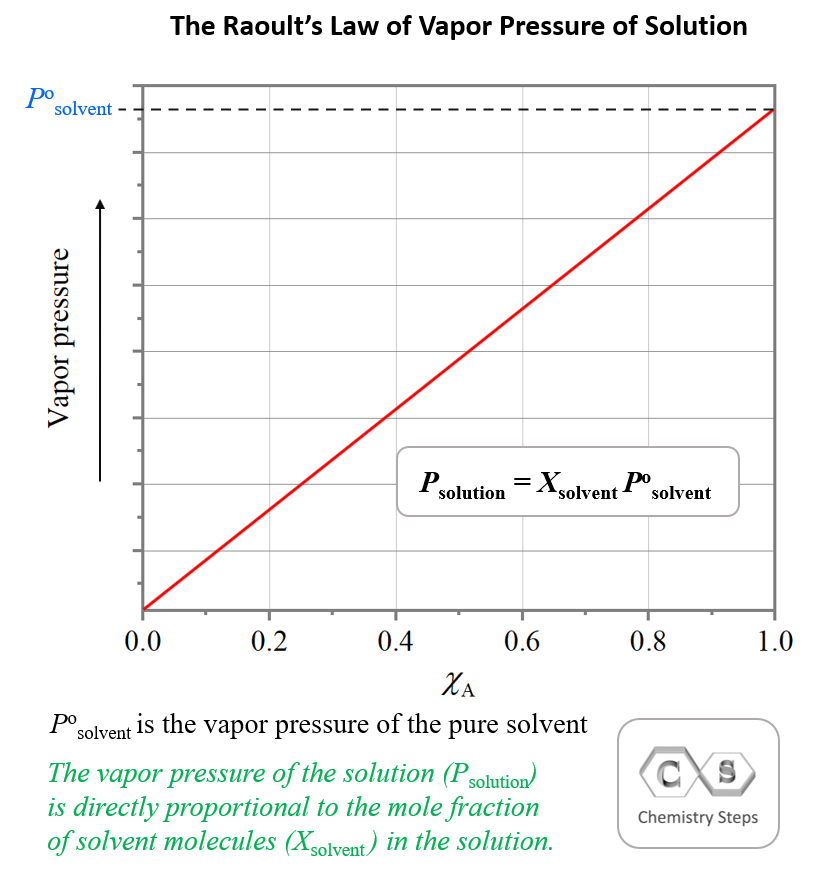
Vapor Pressure Example Problems . Web we can solve vapor pressure problems in either of two ways: Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. By using equation \ref{13.6.1} to calculate the actual vapor. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent.
from general.chemistrysteps.com
By using equation \ref{13.6.1} to calculate the actual vapor. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. Web we can solve vapor pressure problems in either of two ways: Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a.
Vapor Pressure Lowering Chemistry Steps
Vapor Pressure Example Problems Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a. Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web we can solve vapor pressure problems in either of two ways: Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. By using equation \ref{13.6.1} to calculate the actual vapor.
From saylordotorg.github.io
Vapor Pressure Vapor Pressure Example Problems Web we can solve vapor pressure problems in either of two ways: Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web at 15 degrees, the vapor only creates. Vapor Pressure Example Problems.
From mrlvohyvol.blogspot.com
How To Find Vapor Pressure Of A Solution Determine vapor pressure of Vapor Pressure Example Problems [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a. Vapor Pressure Example Problems.
From www.youtube.com
Vapor pressure example Chemistry Khan Academy YouTube Vapor Pressure Example Problems Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. By using equation \ref{13.6.1} to calculate the actual vapor. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web we can solve vapor pressure problems in either of two ways:. Vapor Pressure Example Problems.
From www.youtube.com
How to Solve Vapor Pressure & ClausiusClapeyron Equation Problems Vapor Pressure Example Problems Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces. Vapor Pressure Example Problems.
From jmpcoblog.com
How To Read A Pump Curve Part 2 Vapor Pressure Example Problems Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. By using equation \ref{13.6.1} to calculate the actual vapor. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web at 15 degrees, the vapor only creates a pressure of. Vapor Pressure Example Problems.
From www.ck12.org
VaporPressure Lowering (Raoult's Law) ΔP = X[B]P°[A] Example 1 Vapor Pressure Example Problems Web we can solve vapor pressure problems in either of two ways: Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. Web this example problem. Vapor Pressure Example Problems.
From www.youtube.com
Vapor pressure example Chemistry Khan Academy YouTube Vapor Pressure Example Problems Web we can solve vapor pressure problems in either of two ways: Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. By using equation \ref{13.6.1} to calculate the actual vapor. Web at 15 degrees,. Vapor Pressure Example Problems.
From www.slideserve.com
PPT I. The Vapor Pressure of Solutions PowerPoint Presentation, free Vapor Pressure Example Problems Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong. Vapor Pressure Example Problems.
From quizizz.com
Vapor Pressure and Intermolecular forces Quizizz Vapor Pressure Example Problems Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web we can solve vapor pressure problems in either of two ways: Web at 15 degrees, the vapor only creates. Vapor Pressure Example Problems.
From www.wikihow.com
3 Easy Ways to Calculate Vapor Pressure (with Pictures) Vapor Pressure Example Problems Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. By using equation \ref{13.6.1} to calculate the actual vapor. Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Web we can solve vapor pressure problems. Vapor Pressure Example Problems.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 Vapor Pressure Example Problems [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. By using equation \ref{13.6.1} to calculate the actual vapor. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at. Vapor Pressure Example Problems.
From www.youtube.com
VaporPressure Lowering (Raoult's Law) ΔP = XBP°A YouTube Vapor Pressure Example Problems Web we can solve vapor pressure problems in either of two ways: By using equation \ref{13.6.1} to calculate the actual vapor. Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at. Vapor Pressure Example Problems.
From chem.libretexts.org
13.6 Vapor Pressures of Solutions Chemistry LibreTexts Vapor Pressure Example Problems By using equation \ref{13.6.1} to calculate the actual vapor. Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Web we can solve vapor pressure problems in either of two ways: Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. [ problem. Vapor Pressure Example Problems.
From saylordotorg.github.io
Vapor Pressure Vapor Pressure Example Problems Web we can solve vapor pressure problems in either of two ways: Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a. [ problem 1 (dahm & visco, 2015, w/. Vapor Pressure Example Problems.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Vapor Pressure Example Problems Web the vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. By using equation \ref{13.6.1} to calculate the actual vapor. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web at 15 degrees, the vapor only creates a pressure of. Vapor Pressure Example Problems.
From ar.inspiredpencil.com
Vapor Pressure Example Vapor Pressure Example Problems By using equation \ref{13.6.1} to calculate the actual vapor. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web we can solve vapor pressure problems in either of two ways: Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a. Vapor Pressure Example Problems.
From studylib.net
Vapor Pressure Lowering Example Problem Vapor Pressure Example Problems Web this example problem demonstrates how to use raoult's law to calculate the change in vapor pressure by adding a strong electrolyte to a. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web the vapor pressure of a liquid decreases as the strength of its intermolecular forces increases. By. Vapor Pressure Example Problems.
From www.slideserve.com
PPT Solution properties PowerPoint Presentation, free download ID Vapor Pressure Example Problems Web at 15 degrees, the vapor only creates a pressure of 12.8 mmg hg while at 25 degrees, the vapor creates a pressure. By using equation \ref{13.6.1} to calculate the actual vapor. [ problem 1 (dahm & visco, 2015, w/ permission) estimate the vapor pressure of ethanol at temperatures of 0,. Web we can solve vapor pressure problems in either. Vapor Pressure Example Problems.